Abstract
Introduction: The development of immune checkpoint inhibitors (ICPis) has ushered in a new paradigm for the treatment of both solid and liquid malignancies. However, these therapies, which promote an activated T cell phenotype, can cause a unique spectrum of autoimmune toxicities known as immune-related adverse events (IRAEs), which can affect a variety of organ systems. Autoimmune hemolytic anemia (AIHA) has been described as a rare complication of ICPis, but existing data are mostly limited to isolated case reports. Further, guidelines for the diagnosis and treatment of these patients are lacking. We describe the clinical and laboratory features of 14 patients who developed ICPi-associated AIHA.
Methods: We contacted hematology departments at 18 academic medical centers across the U.S. to inquire about potential cases of ICPi-associated AIHA. Nine centers contributed a total of 14 cases to the current series. We defined AIHA as an abrupt decrease in hemoglobin ≥2g/dL, the presence of at least two laboratory features of hemolysis (elevated serum lactate dehydrogenase [LDH], elevated reticulocyte percentage, low or absent serum haptoglobin, and spherocytes), and the exclusion of other causes of anemia. We collected data on demographics and comorbidities, features of AIHA, and response to treatment. We defined complete recovery (CR) and partial recovery (PR) of hemoglobin as an increase from the nadir hemoglobin to within 1 and 2 g/dL of the baseline value, respectively, in the absence of recent transfusion.
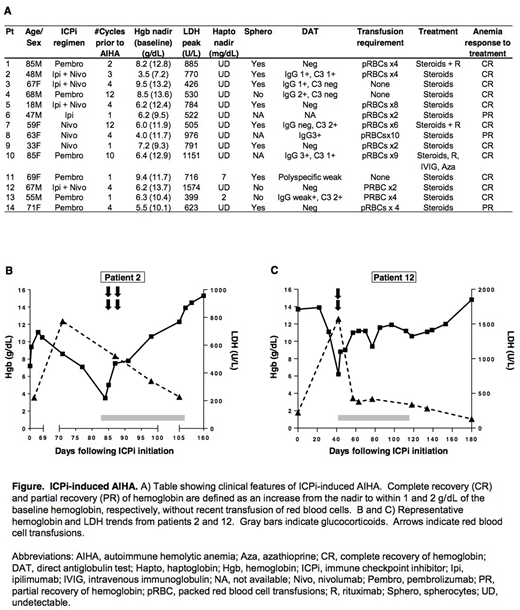
Results: The clinical features of the 14 patients in our cohort with ICPi-associated AIHA are summarized in the Figure (panel A). Underlying malignancies included melanoma (n = 9), non-small cell lung cancer (n = 3), colorectal cancer (n = 1), and acute myelogenous leukemia (n = 1). Four patients had a pre-existing diagnosis of a lymphoproliferative disorder, and two of these patients were known to have a positive direct antiglobulin test (DAT) prior to initiation of ICPi. ICPis used included ipilimumab, nivolumab, a combination of ipilimumab and nivolumab, and pembrolizumab.
Representative hemoglobin and LDH trends are shown in the Figure (panels B and C). The median (interquartile range [IQR]) interval from initiation of ICPis to AIHA was 55 (22-110) days. Eight patients (57%) were DAT positive: 3 were positive for both IgG and C3; 3 were positive for IgG alone; 2 were positive for C3 alone; and 1 was weakly positive on polyspecific testing. Five patients (36%) were DAT negative, and in one patient a DAT was not performed. The median (IQR) hemoglobin nadir was 6.3 (6.1-8.0) g/dL,and 11 patients (79%) required red blood cell transfusion. All patients were treated with glucocorticoids, with 3 requiring additional immunosuppressive therapy.
All patients achieved a CR or PR of hemoglobin in response to treatment (CR, n = 11; PR, n =3). The median (IQR) time from the hemoglobin nadir to CR or PR was 47 (30-62) days. Seven patients (50%) experienced at least one other IRAE. Seven patients (50%) were re-challenged with ICPis, and one patient developed recurrent AIHA with re-challenge.
Conclusion: ICPi-associated AIHA is a recently-described phenomenon, which will be encountered more frequently with the widespread use of ICPis. ICPi-associated AIHA shares many clinical features with other forms of AIHA; however, a unique aspect of ICPi-associated AIHA appears to be a relatively high incidence of DAT negativity (36% in our series, compared with 3-11% in other forms of AIHA). It is possible that more advanced DAT testing - i.e., "super-Coombs" - which was not performed in any of the patients in this series, would have yielded different results.
Glucocorticoids appear to be an effective first-line regimen in most patients with ICPi-associated AIHA. Furthermore, we found that AIHA does not necessarily recur with ICPi re-challenge. Additional studies are needed to better understand the mechanisms underlying ICPi-associated AIHA and optimal treatment strategies.
Anagnostou:ASH Research Training Award for Fellows 2018: Research Funding; Mayo Clinic SPORE: Research Funding; Mayo Clinic Immune Monitoring Core: Research Funding; Mayo Clinic Hematology Research Division: Research Funding. Salama:BMS: Research Funding; Celldex: Research Funding; Dynavax: Research Funding; Genentech: Research Funding; Merck: Research Funding; Reata: Research Funding; Incyte: Research Funding; Immunocore: Research Funding; Array: Consultancy; Merck: Consultancy; Biocryst: Consultancy; BMS: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal